What is the number of core and valence electrons for I?
2 Answers
- Valence electrons are the total number of electrons present in the outermost shell of an atom (i.e. In outermost orbital). The valence electrons for a neutral atom is always definite, it cannot be varied (more or less) in any condition for a particular atom and may or not be equal to its valency.
- Comprehensive information for the element Iodine - I is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions.
Explanation:
Valence Electrons The electrons in the last orbit which also determines mainly the electrical properties of the elements are known as valence electrons. It is well known to us that the outermost shell of an atom processes maximum 8 number of electrons. So the maximum number of valence electrons of an atom cannot be more than 8. Valence electrons are those electrons found in the outermost shell of an atom. In other words, these are the electrons that can be gained or lost during a chemical reaction. Where are valence electrons located?
Iodine,
That means that a neutral iodine atom will have a total of
Now, out of these
But how would you distinguish between the two types of electrons?
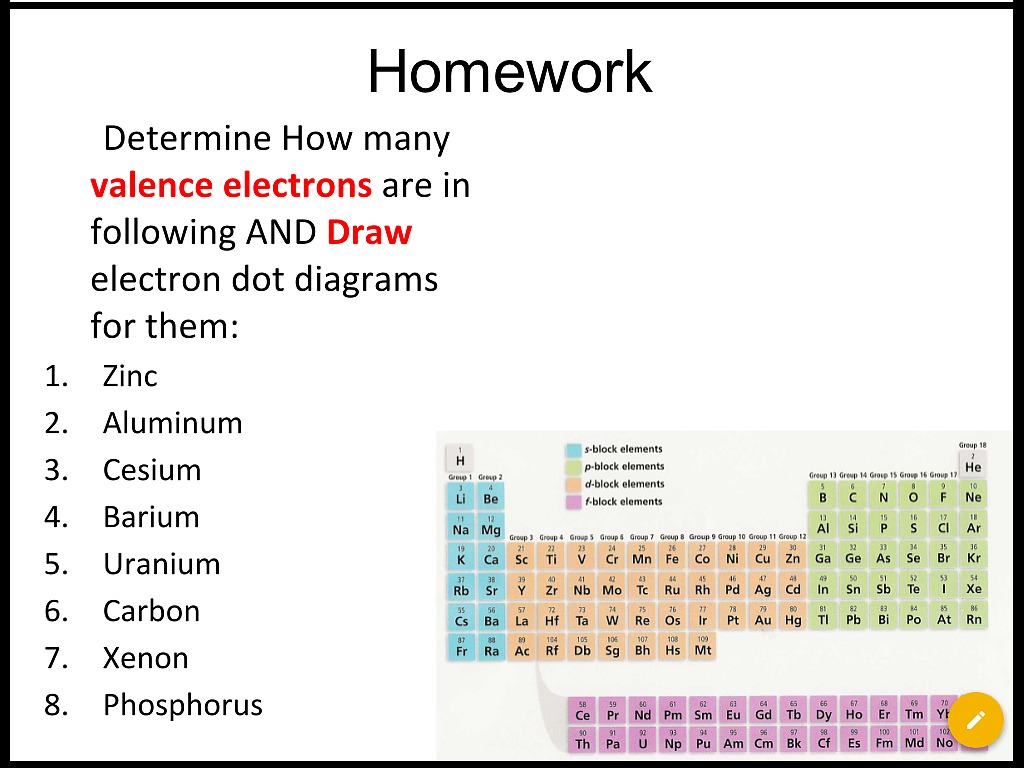
To do that, you need to look at the electron configuration of iodine, which looks like this
You know that the valence electrons are defined as being the electrons located on the highest energy level of that atom, which is given by the principal quantum number,
Notice that, in iodine's case, the highest energy level is
This means that iodine has a total of
The rest of the electrons will thus be core electrons.
Therefore, a neutral iodine atom has
Iodine has
Explanation:
Iodine is in the group 17 elements, known as halogens, and all of them have
A valence electron is capable of bonding with valence electrons of another atom to complete the octet of electrons each atom needs in its outer shell.


Valence Electrons Iodine
The core electrons are not available for bonding with other elements
When more than one atom bonds with others, a molecule is formed. The completed molecule will not have an electron charge associated with it.
Related questions
Learning Objectives
- Define valence electron.
- Be able to indicate valence electrons when given the electron configuration for an atom.
What makes a particular element very reactive and another element non-reactive?
A chemical reaction involves either electron removal, electron addition, or electron sharing. The path a specific element will take depends on where the electrons are in the atom and how many there are.
| Element Name | Symbol | Atomic Number | Electron Configuration |
| Lithium | Li | 3 | 1s22s1 |
| Beryllium | Be | 4 | 1s22s2 |
| Boron | B | 5 | 1s22s22p1 |
| Carbon | C | 6 | 1s22s22p2 |
| Nitrogen | N | 7 | 1s22s22p3 |
| Oxygen | O | 8 | 1s22s22p4 |
| Fluorine | F | 9 | 1s22s22p5 |
| Neon | Ne | 10 | 1s22s22p6 |
In the study of chemical reactivity, we will find that the electrons in the outermost principal energy level are very important and so they are given a special name. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements listed above, the two electrons in the 1 s sublevel are called inner-shell electrons and are not involved directly in the element’s reactivity or in the formation of compounds. Lithium has a single electron in the second principal energy level and so we say that lithium has one valence electron. Beryllium has two valence electrons. How many valence electrons does boron have? You must recognize that the second principal energy level consists of both the 2 s and the 2 p sublevels and so the answer is three. In fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. Neon, with its configuration ending in s2p6, has eight valence electrons.
Summary
- Valence electrons are the outer-shell electrons of an atom.
- Valence electrons determine the reactivity of an atom.
Practice
Use the link below to answer questions about valence electrons:
Review
- Define valence electron.
- Define inner shell electron.
- How many valence electrons are there in fluorine?
- What are the 2s electrons in nitrogen?
- How many inner shell electrons are there in beryllium?
Glossary
- inner-shell electrons: Those electrons that are not in the outer shell and are not involved in the reactivity of the element.
- valence electrons: The electrons in the highest occupied principal energy level of an atom.
References
F Cl. Br And I Valence Electrons
- User:Chemicalinterest/Wikipedia. http://commons.wikimedia.org/wiki/File:Cobalt_carbonate.JPG.
